
+86-512-82868386

+86-512-82868386
Product Center
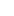 Product
Product
Influenza A/B Virus/ COVID-19 Antigen Combined Rapid Test Kit
Influenza A/B Virus/ COVID-19 Antigen Combined Rapid Test Kit

product details
/ PRODUCT DETAILS
【Packing specification】
5 Tests/Kit、25 Tests/Kit、50 Tests/Kit
【Expected usage】
COVID-19 Antigen/Influenza A+B Antigen Combo Detection Kit is a lateral flow immunoassay for the qualitative detection of SARS-CoV-2 nucleocapsid antigens and Influenza A and B virus antigens in throat swabs and nasopharyngeal swab samples from individuals suspected of COVID-19 and Flu A/B.
Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out other bacterial or viral infections.
Negative results from patients with symptom onset beyond seven days should be confirmed with a molecular assay. Negative results do not rule out SARS-CoV-2 infection or Influenza A/B infection and should not be used as the sole basis for treatment or patient management decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19 and Influenza A/B.
The product is intended to be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulations. For in vitro diagnostic use only.
【Detection principle】
This kit uses the principle of colloidal gold immunochromatographic double antibody sandwich method to qualitatively detect the SARS-CoV-2 nucleocapsid antigens and Influenza A and B virus antigens in human throat swabs and nasopharyngeal swabs.
During the test, specimens and detection buffer are applied to the test cartridges. If there are SARS-CoV-2 nucleocapsid antigens or influenza A/B virus in the specimens, they will bind to colloidal gold-labeled antibodies against SARS-CoV-2 N protein or Influenza A and B virus antigens respectively on conjugation pad forming virus antigen-antibody-colloidal gold complexes (complex A for Flu A, complex B for Flu B and complex C for COVID-19 ).
During lateral flow, the complexes move along nitrocellulose membrane toward one end of the absorbent paper. When passing the COVID-19 test line TC (coated with another monoclonal antibody against SARS-CoV-2 N protein), the complex C is captured by capture antibody resulting in coloring on line TC; when passing the Influenza A test line TA (coated with another monoclonal antibody against Influenza A virus N protein), the complex A is captured by capture antibody resulting in coloring on line TA; when passing the Influenza B test line TB (coated with another monoclonal antibody against Influenza B virus N protein), the complex B is captured by capture antibody resulting in coloring on line TB; when passing the line C, residual colloidal gold-labeled control molecule is captured by quality-control antibody resulting in coloring on line C.